s en quelques minutes, où que tu sois


Des fonctionnalités intelligentes qui vous facilitent la vie

Tout ce dont tu as besoin dans un seul kit
Rapide. Simple.
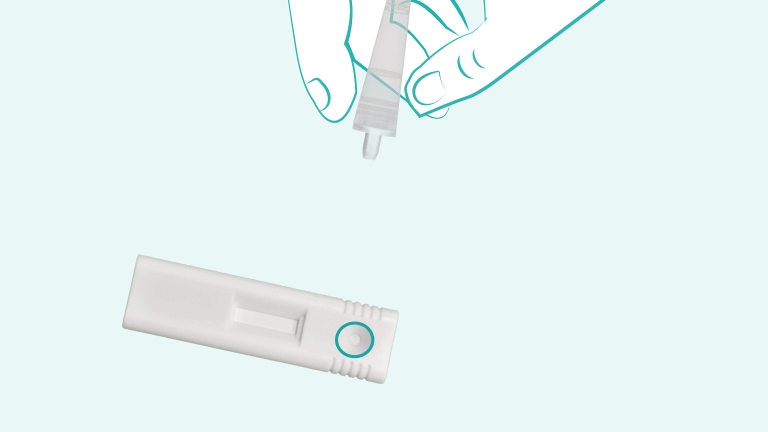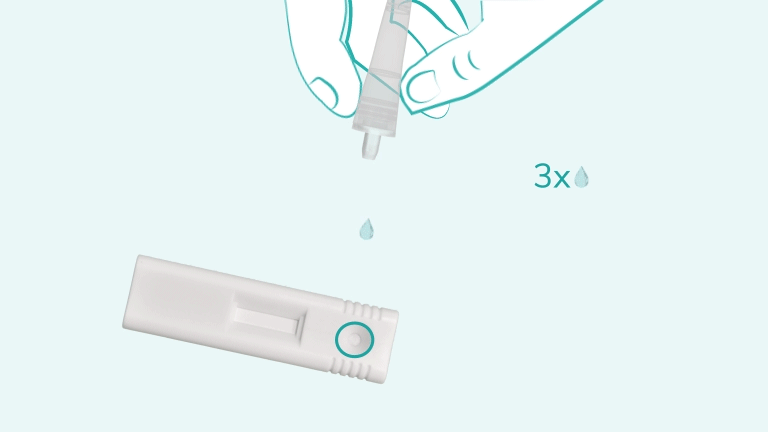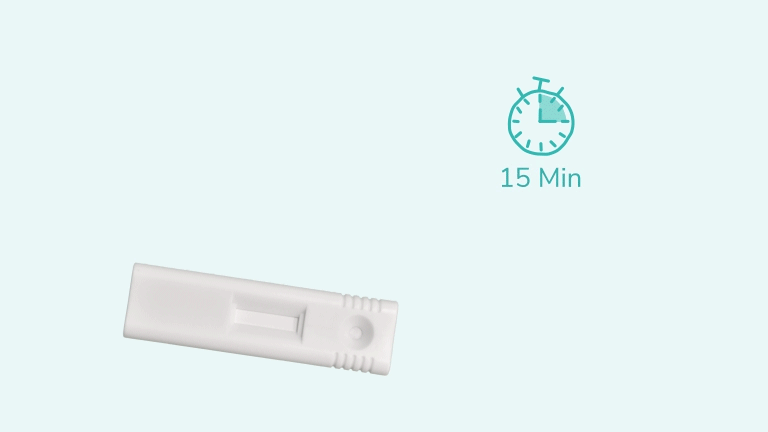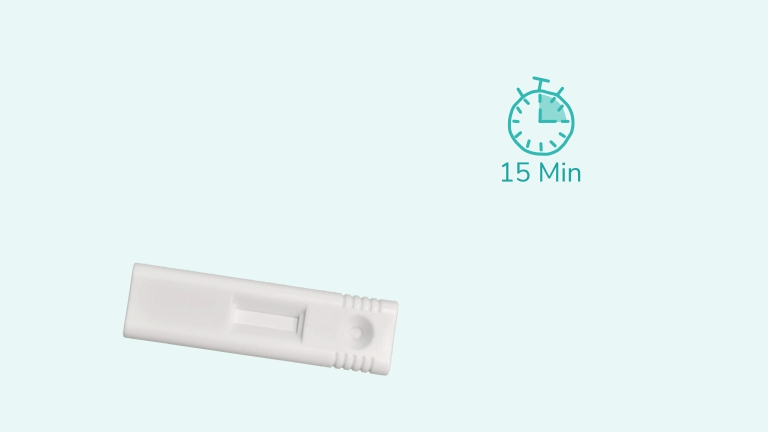
C'est fait en 3 étapes.
Réponses à vos questions
The iStatis COVID-19 Antigen Home Test is a rapid, single-use diagnostic tool designed to detect the presence of proteins (antigens) from the SARS-CoV-2 virus in the nose. It uses a gentle anterior nasal swab and provides visually readable results in 15 minutes. The test is intended for use at home by individuals aged 15 years and older, or by an adult administering it to children aged 2 to 14 years.
The test can be used by individuals who are 15 years or older for self-collection. For children between 2 and 14 years old, the test must be performed and interpreted by a parent or guardian. The test is not suitable for anyone under the age of 2.
You should use the test if you are experiencing symptoms of COVID-19 and are within the first seven days of symptom onset. It is also appropriate to use the test if you are asymptomatic but suspect exposure to COVID-19. In these cases, the test should be taken twice over two to three days, with at least 24 hours and no more than 48 hours between tests.
Common symptoms of COVID-19 include fever, cough, shortness of breath, fatigue, loss of taste or smell, headache, sore throat, muscle aches, chills, nausea, vomiting, or diarrhea. These symptoms can appear any time from two to fourteen days after exposure to the virus. In some cases, infected individuals may not show any symptoms at all.
The nasal swab provided is soft and not sharp. Most people find it only slightly uncomfortable. If you experience pain during the swabbing process, stop the test and consult a healthcare provider.
Each test has a built-in control line that appears on the cartridge if the test has been performed correctly and the sample was sufficient. If the control line does not appear, the test is invalid. In this case, you must repeat the test with a new kit.
If both the control line and the test line are visible, the result is positive, indicating that SARS-CoV-2 was found in your sample. You should immediately isolate and contact your healthcare provider for follow-up testing. If only the control line is present, the result is negative, meaning the virus was not detected. If no control line appears, regardless of the presence of a test line, the result is invalid and cannot be interpreted.
While the test is highly accurate, no diagnostic test is perfect. A negative result may still occur even if you have COVID-19, especially if the viral load is too low to be detected or if the sample was not collected properly. A positive result is generally reliable, but in rare cases, false positives can occur. If your results are unexpected or you remain concerned, consult your healthcare provider and consider follow-up testing with a molecular (PCR) test.
The buffer solution in the vial contains 0.1% sodium azide, which is harmful if swallowed. It should not be ingested and must be kept away from children. Avoid contact with the skin and eyes. If contact occurs, rinse thoroughly with water and seek medical advice if irritation continues.
After completing the test, return all components to the original packaging and dispose of them in a standard household waste bin. Follow any applicable local regulations for waste disposal.
If you have any questions or need technical assistance, you can contact bioLytical Technical Support at 1-866-674-6784 or email customercare@biolytical.com.


