Rapid, Reliable Results for Informed Clinical Decisions


Smart Features That Work for You

Everything You Need in One Kit
Fast. Simple.
Done in 3 Steps.
Your Questions, Answered
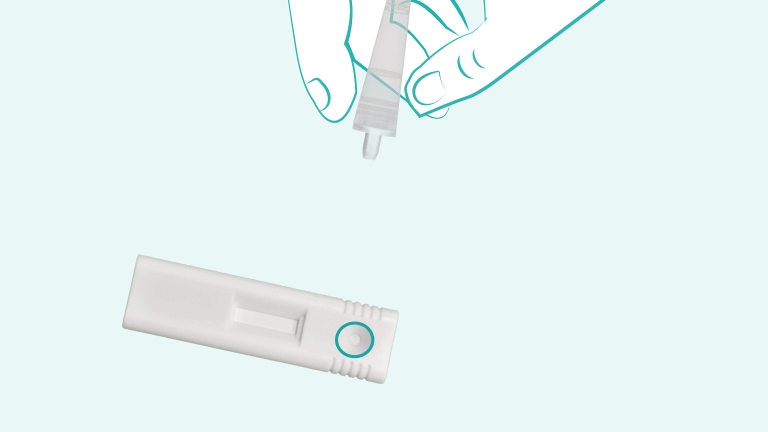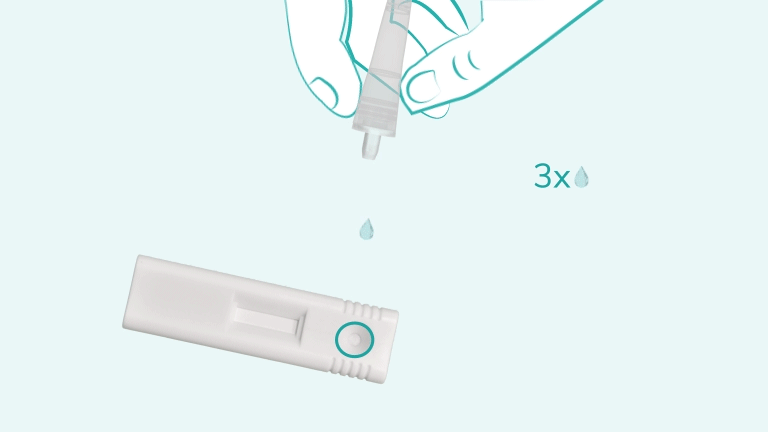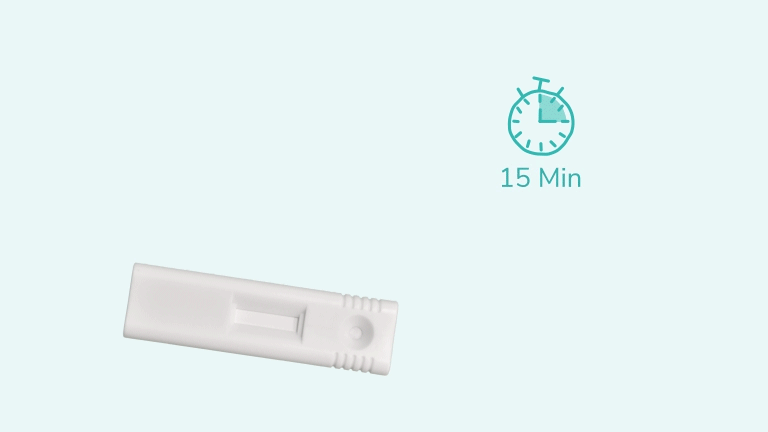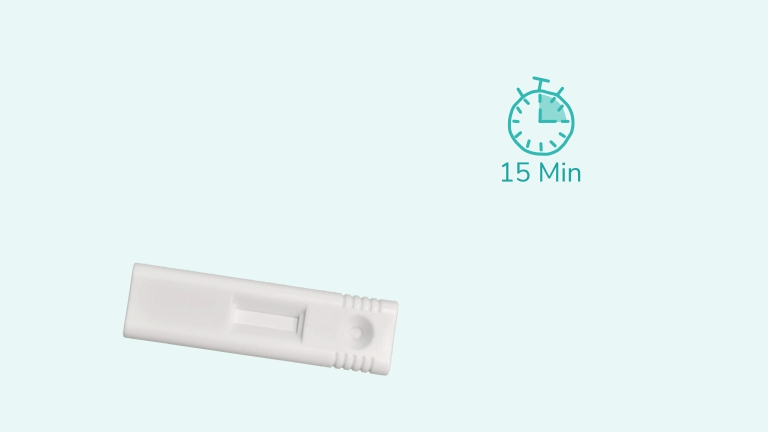
It is a single-use, lateral-flow immunoassay designed to detect nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens. Results are visually interpreted and available within 15 minutes
The test is intended for use by trained healthcare professionals or operators in near-patient or point-of-care (POC) settings
Test results should be read at 15 minutes and no later than 30 minutes after adding the sample to the cartridge
A control line and any visible test line (even faint) indicate a positive result.
A visible control line and no test line indicate a negative result.
The result is invalid. Retest with a new cartridge
Yes, but only as part of serial testing (twice over 2–3 days with 24–48 hours between tests). Confirmatory molecular testing may be needed, especially in cases of high exposure risk
Discard the vial and use a new kit.
Store at 2°C to 30°C (35.6°F to 86°F). Ensure the kit is at room temperature before use
No, all components are single-use only.
No. Negative results do not exclude infection. If symptoms persist or exposure is suspected, confirm with a molecular test


