Screen for Hepatitis—Fast. Reliable. Where It Counts Most.


Smart Features That Work for You
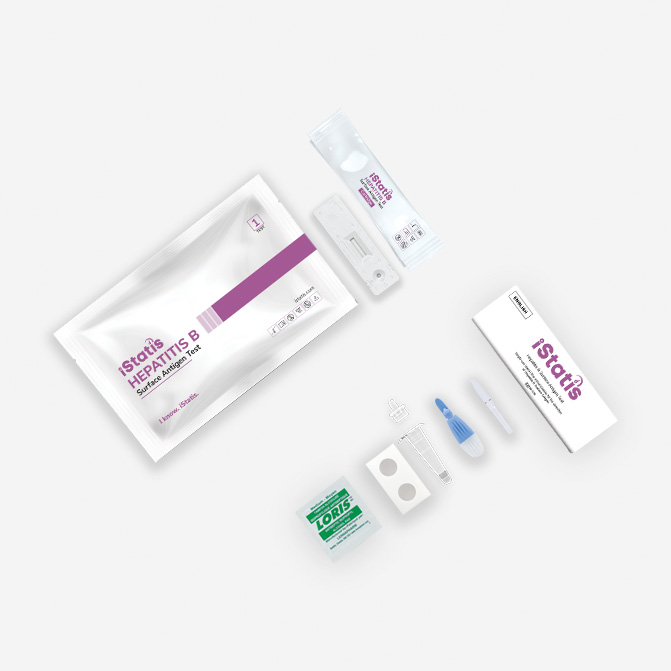
Everything You Need in One Kit
Fast. Simple.
Results in 3 Steps.




The iStatis HBsAg Test is a rapid, single-use lateral flow immunoassay designed to detect Hepatitis B Surface Antigen in human EDTA-whole blood, fingerstick blood, serum, or plasma. It provides results within 15 minutes and is intended for use by healthcare professionals in clinics, laboratories, and point-of-care settings. It is not intended for home use
This test is authorized for use on adults aged 18 years or older. The performance of the device has not been established in individuals under 18
The test uses antibodies to detect the presence of Hepatitis B Surface Antigen (HBsAg) in a blood sample. When a sample is applied, it reacts with antibodies on the strip. A positive result shows a blue line at the test position “T” along with a control line “C”. A negative result shows only the control line. The test should be read at 15 minutes and no later than 30 minutes
The iStatis HBsAg Test can be performed using fingerstick whole blood, venous whole blood collected in EDTA tubes, serum, or plasma. A 50 µL sample volume is required. Plasma or serum should be separated and stored appropriately before testing
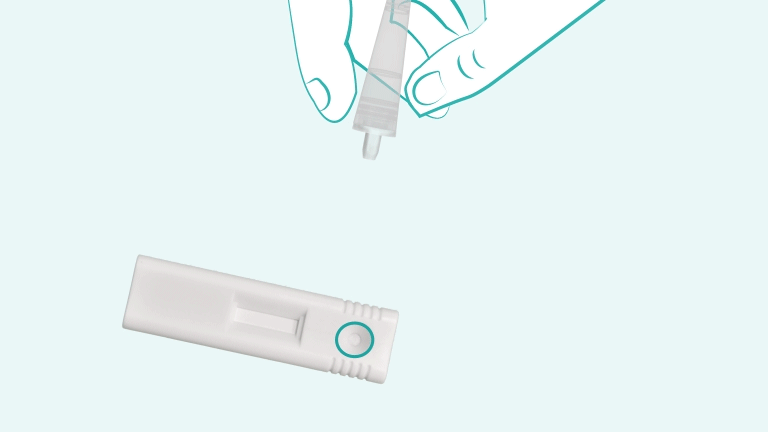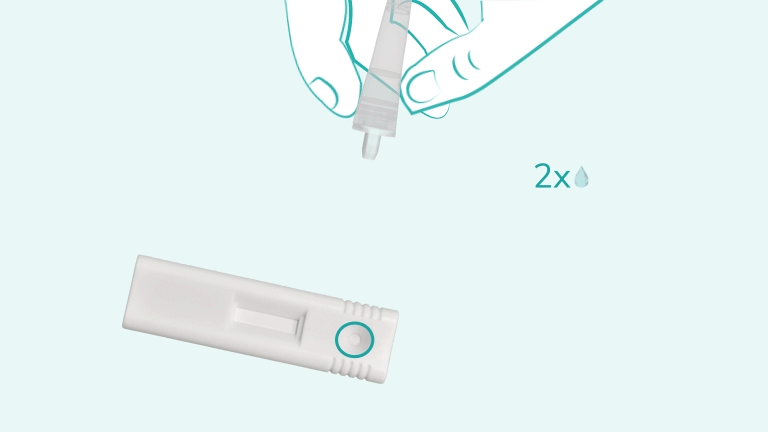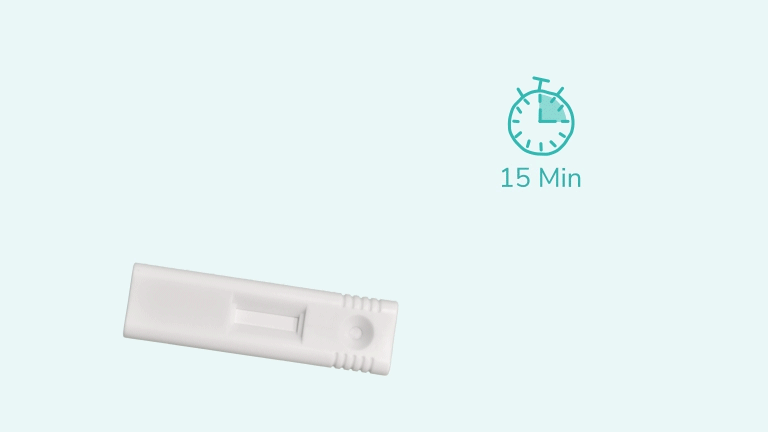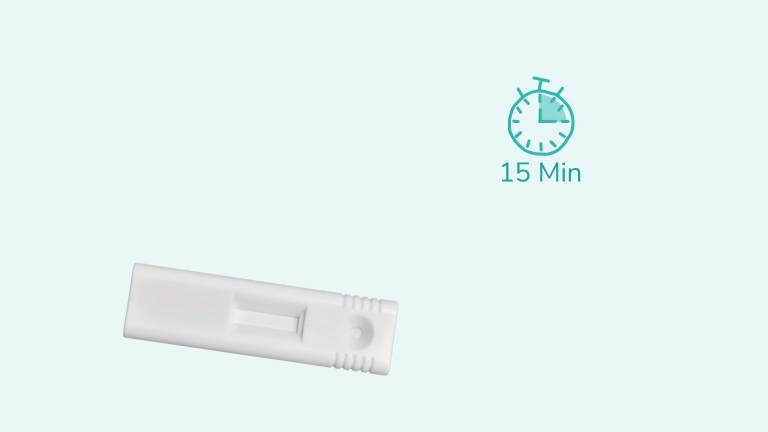
First, clean the fingertip with the alcohol swab included in the kit. Use the sterile lancet to prick the finger, then use the provided capillary pipette to collect blood up to the fill line. Dispense the blood into the cartridge sample well, add 2 drops of chase buffer, and start the timer
A control line must appear at the “C” position of the cartridge. This indicates that the sample and buffer have flowed correctly. If no control line appears, the test is invalid and must be repeated using a new test kit
A positive result shows blue lines at both the control (C) and test (T) areas, indicating the presence of HBsAg in the sample. A negative result shows a blue line at the control area only. An invalid result means no control line is visible, regardless of the test line. Repeat the test if the result is invalid
If your test result is positive, a venous blood sample must be collected in an appropriate tube and sent to a laboratory for confirmatory testing. Follow all applicable pre- and post-test counseling procedures
In clinical studies, the iStatis HBsAg Test demonstrated excellent accuracy across all sample types. For fingerstick capillary blood, the test showed 100% Positive Percent Agreement (PPA) and 100% Negative Percent Agreement (NPA). For venous whole blood, serum, and plasma, the PPA was 99.5% and the NPA was 100%
No interference was observed from common infectious diseases (like HIV, HCV, influenza) or from conditions like autoimmune diseases, pregnancy, or use of common medications. However, very high levels of interfering substances or improper handling may impact results
Yes. The chase buffer contains 0.08% sodium azide, which is harmful if swallowed. Avoid contact with skin or eyes, and keep out of reach of children. Dispose of the used vial according to local hazardous waste regulations